Fueled by nanoparticles, new catalyst does more with less platinum
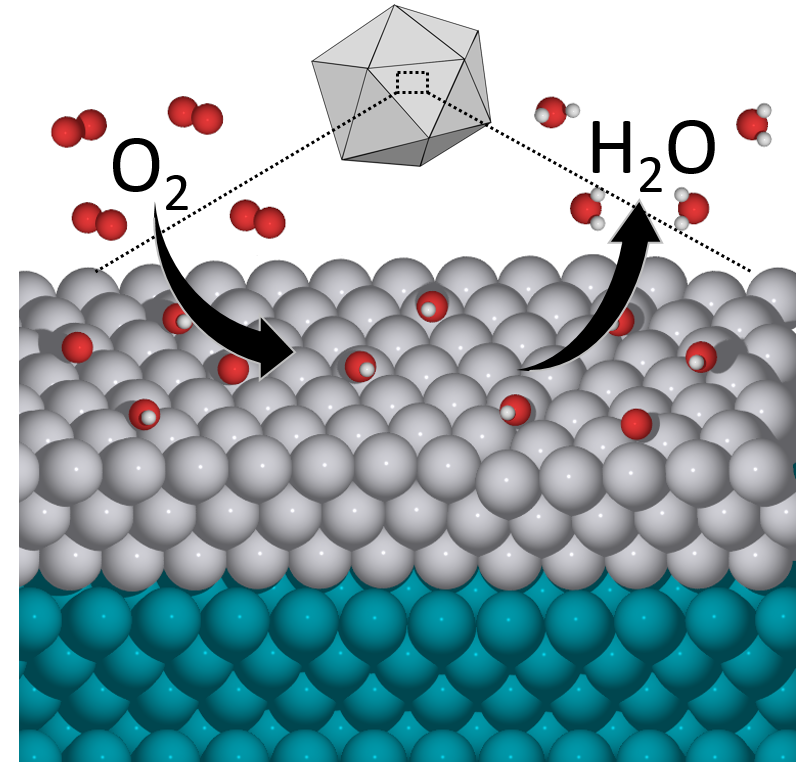
A chemical diagram shows the oxygen reduction reaction, a key chemical reaction in many energy and chemical processes, taking place with an icosahedral particle made of palladium and platinum. (Credit: Mavrikakis group, UW–Madison)
Platinum is a highly reactive and in-demand catalyst across the chemical and energy industries, but a team of University of Wisconsin–Madison and Georgia Institute of Technology scientists could reduce the world’s dependence on this scarce and expensive metal.
In a paper published July 2 in the journal Nature Communications, Professor Manos Mavrikakis and his research group describe a new catalyst that combines platinum with the less expensive metal palladium. This not only reduces the need for platinum but actually proves significantly more catalytically active than pure platinum in the oxygen reduction reaction, a chemical process key to fuel cell energy applications. The palladium-platinum combination also proves more durable, compounding the advantage of getting more reactivity with less material. Just as importantly, the paper offers a way forward for chemical engineers to design still more new catalysts for a broad range of applications by fine-tuning materials on the atomic scale.
The discovery was set in motion last year, when researchers at Georgia Tech developed nanoparticles consisting mostly of palladium, with a relatively small amount of platinum worked into the surface. Early on they realized that these particles showed significantly greater activity than pure platinum, as measured by dividing the current produced by the oxygen reduction reaction by the mass of platinum used. But to understand the why-the chemistry driving this apparent advantage-the Georgia Tech researchers turned to the UW–Madison team of Mavrikakis, graduate student Luke Roling and postdoctoral researcher Jeffrey Herron. Mavrikakis is the Paul A. Elfers Professor and Vilas Distinguished Achievement Professor of Chemical and Biological Engineering.
Roling says that from a chemist’s point of view, the new catalyst’s high performance at first seemed counterintuitive. But the Mavrikakis group, by applying its strengths in modeling and computational analysis, discovered that the advantage lays in the nanoparticle’s icosahedral, or 20-faceted, structure. Moving forward, researchers looking for new catalysts can experiment with similar structures and perhaps find even more reactive materials.
For Mavrikakis, the results vindicate years of research-on both the theoretical and synthesis sides of catalysis-that has focused on the importance of how a particular substance’s reactivity changes depending on whether it’s structured as an icosahedron, an octahedron or another shape.
“This is speaking to the precise arrangement of atoms on the surface of a nanoparticle,” Mavrikakis says. “That can make an enormous difference in how fast the reaction takes place. Theory has been instrumental for about 10 years now to demonstrate the importance of being able to tailor-make specific facets of the same material.”
As rapidly improving technology to synthesize new tailor-made materials aligns with quantum mechanics, this research could make all manner of catalysis-driven processes more efficient and less expensive.
“The goal here is to try to minimize the amount of platinum that you use, and eventually find a complete replacement of platinum,” Mavrikakis says. “If we can move away from platinum, many of these applications have the potential to become more robust financially.”
The Mavrikakis group’s research was supported by the Department of Energy and funds from the Vilas Distinguished Achievement Professorship.
###
-Scott Gordon, (608) 265-8592, gordon@engr.wisc.edu
