Waisman Center: Celebrating 40 years of advancing knowledge about developmental disabilities

From her perch as director of the Waisman Center, and with an insider’s knowledge of its work to advance our understanding of developmental disability and the people it affects, Marsha Mailick sees a hopeful microcosm of the best attributes of the University of Wisconsin–Madison.

Although its roots are deeper, going back to its earliest iteration as the Joseph P. Kennedy, Jr. Memorial Laboratories in the early 1960s, the Waisman Center this year celebrates 40 years of research, teaching and outreach in the interest of developmental disabilities. Exploring the range of disabilities, the Waisman Center community has helped gather new knowledge on the basic biology, developmental course, prevention, treatment and social context of a highly complex set of human conditions.
This is no small thing. Developmental disability, which can be cognitive or physical, is estimated by the Centers for Disease Control to affect as many as 1 in 6 children under the age of 17 in the United States. Such conditions run the gamut from autism and Down syndrome to the kinds of intellectual deficits once referred to as mental retardation. Generally, such conditions are expected to last a lifetime.
“Developmental disability and our understanding of its causes is one of the great research quests of our day,” explains Mailick. “We know we have had an impact on the health and quality of life of many thousands of people over the course of our existence. Our hope and intent is to continue that tradition, helping children and adults with developmental disabilities and their families live full, healthy and meaningful lives and to seek new knowledge about the causes, consequences, and treatments for these conditions.”
Those helped by the Waisman Center, such as Claire Bible, who has a mild form of Down syndrome (mosaic), knows the difference that the center’s work can make. She recently donated a skin sample to be used in research by Waisman scientist Anita Bhattacharyya, to develop a Down syndrome induced pluripotent stem cell line.

Bible
“There are many ‘what ifs’ in the world,” Bible says. “I wondered how did I come into the world with mosaic Down syndrome? I began thinking about the role of environment and genetics. I hoped that by donating a skin sample, I could help answer some of the ‘what ifs’ about mosaic Down syndrome for future generations.”
The center is named after Harry Waisman, the pioneering Wisconsin pediatrician and biochemist whose work helped inform our understanding of Phenylketonuria or PKU, an inherited form of mental retardation, and its prevention. Like Waisman’s early work, the basic science conducted at the center has helped identify key risk factors for developmental disability as well as treatments, and has also shed light about the genetic and environmental causes that give rise to such conditions.
Waisman’s scientific passion and advocacy for those with developmental disorders, notes Mailick, is the archetype for what has become one of the world’s leading centers of research, education and outreach on developmental disabilities and neurodegenerative diseases.
Forty-plus years of discovery, education and patient outreach are impossible to catalog in any simple account of the center. But for starters, we share 10 signature accomplishments that have made the world better and serve as examples of what lies ahead.
More Effective Treatment for PKU
Mandatory screening for and treating Phenylketonuria was one of Harry Waisman’s signature missions in life. Untreated, PKU leads to profound mental retardation. If the condition is identified at birth, it can be effectively treated through a special diet that helps patients mitigate the toxic effects of an amino acid that those with PKU are unable to metabolize. But the diet, explains Mailick, leaves much to be desired as it is unappetizing and does not satisfy hunger. The diet is a lifelong exercise to keep PKU at bay and pregnant women with PKU must conform to the diet to prevent their babies from having the condition.
Enter Waisman researchers Denise Ney, professor of nutritional science, and Sandra van Calcar, assistant professor of pediatrics, as well as Mark Etzel. Etzel, a professor of food science at UW–Madison, developed a new, more appetizing food for PKU patients, which is now in clinical trials at the Waisman Center and at the Harvard affiliated Children’s Hospital in Boston.
“Denise’s research carries forth the legacy of Harry Waisman through prevention of disability via screening, early identification and dietary treatment,” says Mailick. “Indeed, PKU is the only genetic disorder that can be fully treated.”
The Genetic Roots of Developmental Disability

Professor Albee Messing, left, discovered the gene that causes a rare condition in young children and later devised a test for the disease.
Photo: Jeff Miller
Since Waisman’s death in 1971, there has been “a surge of discovery” in genetic research with well more than six thousand single-gene disorders identified as the root cause of human disease, including many that cause developmental disabilities, according to Mailick. Emblematic of such research is Waisman scientist Albee Messing’s discovery of the gene that causes Alexander disease, a rare but fatal condition that mainly affects young children. Messing, a professor of comparative biosciences in the UW–Madison School of Veterinary Medicine, has since devised a genetic test for the disease and begun teasing out the molecular mechanisms that cause symptoms and death in Alexander patients. He is currently screening existing drugs already approved for human use that may help ease the manifestations of this currently untreatable disease.
Fragile X
A condition involving changes in the X chromosome, fragile X is the most prevalent inherited developmental disability. It is caused by changes in a gene known as FMR1 where a small part of the gene’s amino acid sequence repeats more often than normal. In most people, the segment repeats between 5 and 40 times. Increased repetition of the sequence is associated with impaired cognitive and reproductive functions. While 200 repeats is considered a full mutation of the FMR1 gene, anything between 41 and 54 repeats, just above normal expression, falls into what researchers call the “gray zone” where there is a borderline risk of neurological symptoms. Between 55 and 200 repeats is considered a “premutation.” Mailick conducted the first U.S. survey of the prevalence of both the fragile X gray zone and premutation and found a surprisingly high prevalence of both, with the gray zone occurring in one of every 12 females and one of every 21 males, suggesting that the genetic preconditions for clinical fragile X are widespread.
Stem Cells
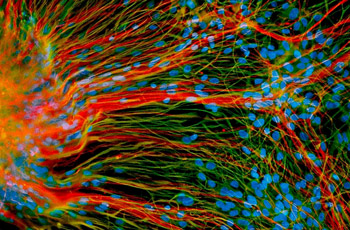
UW-Madison work with stem cells is leading to an understanding of what can go wrong at the level of a cell to cause conditions such as Down, fragile X and Rett syndromes.
Photo: Su-Chun Zhang
First tamed in 1998, embryonic stem cells – the blank slate cells that arise in early development and go on to form all of the 220 tissues in the human body – have since been directed in the lab dish by Waisman Center scientist and Professor of neuroscience Su-Chun Zhang to become some of the most fundamental building blocks of the brain. Scientists can now make neurons and astrocytes with relative ease and they’re hard at work exploring the intricate workings of those cells and how they might be used clinically to alleviate conditions like Parkinson’s and Lou Gehrig’s disease. What’s more, embryonic and now induced pluripotent stem cells are being used by Waisman scientists to create models of diseases like Down, fragile X and Rett syndromes as well as disorders that cause blindness and deafness. Such work promises a fundamental understanding of what goes wrong at the level of the cell to cause those conditions and may one day lead to their prevention.
The Epidemiology of Autism
Since it was first described scientifically in 1943, autism has become recognized as one of the most widespread developmental disorders in the United States and beyond. At the time the Waisman Center was founded, the first epidemiologic study of autism in the United States was underway in Wisconsin under the directorship of Darold Treffert. That study found many children with autism to be institutionalized and without access to public education in the least restrictive environment. It also found the prevalence of autism to be just 3.1 per 10,000 or 1 in 3,225 children.
Collaborative studies now underway at the Waisman Center find the prevalence of autism spectrum disorders in Wisconsin today to be about 7.6 per 1,000 children (1 in 131), or more than 20 times more common than it was found to be in the 1960s.
While the causes of autism and its rising prevalence are the subjects of debate, a number of notable facts and clues to the etiology of the condition have come to light through the work of Waisman Center researcher and professor of population health sciences Maureen Durkin. In carrying out epidemiological investigations of autism prevalence, Durkin and her colleagues identified higher socioeconomic status as a risk factor, whereas the risk of developmental disabilities overall tends to be associated with lower socioeconomic status. Other risk factors for autism identified by Durkin and her colleagues include advanced maternal and paternal ages, and earlier birth order. Much additional research is needed to translate these epidemiological studies into clinical treatments, but understanding the population trends in a necessary first step.
Seeing the Autistic Brain
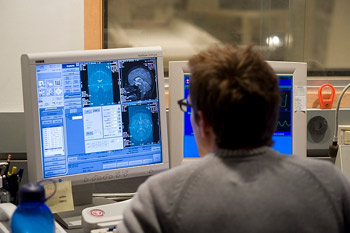
A technician reviews computer displays following an imaging test at the Waisman Center to measure a subject’s brain activity.
Photo: Jeff Miller
Although a prevalent condition, autism remains a mystery on many levels. Waisman Center researcher and Vilas Professor of Psychology and Psychiatry Richard Davidson, using sophisticated brain imaging techniques, has helped resolve a little bit of that mystery by identifying in the amygdala, an almond-sized mass deep in the brain, abnormalities characteristic of autism. Associate Professor of medical physics and psychiatry and Waisman Center researcher Andrew Alexander and his newly-arrived colleague Professor Janet Lainhart of the department of Psychiatry, have shown that a sophisticated form of magnetic resonance imaging can be used to differentiate the brains of individuals with autism and controls with 94 percent accuracy.
Language as Child’s Play

Professor Jenny Saffran’s research is exploring how infants develop language skills.
Photo: Jeff Miller
How infants develop language skills has been the subject of intellectual dogfights for decades. Are we hard wired at birth to acquire language or is language something the brain must soak up from our environment? Psychology Professor Jenny Saffran, who directs the Waisman Center’s Infant Learning Laboratory, waded into the infant language wars as a graduate student and her work then helped show that the language skills of very young humans is, in part at least, due to sophisticated learning strategies that play out in the first year of life. Infants, Saffran found, could sort out and focus on new words embedded in strings of nonsensical language. In the Infant Learning Lab, Saffran and her team continue to divine the mysteries of how we acquire language skills early on and how, as infants, we might become better processors of language.
Speaking Through Computers
Computers help us do many things, but they have become an essential communication lifeline for many children and others with developmental disabilities. Established at the Waisman Center in 1971 to address the needs of people with disabilities who have speech and mobility limitations,, the Trace Center became an innovative player in the emerging field of “augmentative communication.” With the advent of personal computers, the Trace Center helped devise, test and gain industry acceptance of many of the accessibility features that are now standard in the machines we use in our everyday lives. The Trace Center, now a part of the College of Engineering, continues to provide services through the Communication Aids and Systems Clinic at Waisman. The clinic is led by Associate Professor of communicative disorders Katie Hustad.
Growing Old with Down Syndrome
Eighty years ago, people with Down syndrome lived, on average, to 9 years of age. Today, they live into their 50s and 60s thanks to advances in medicine. However, people with Down syndrome tend to exhibit premature aging and dementia. Alzheimer’s, in particular, commonly begins to manifest itself by the fourth decade of life, although many do not show the changes in behavior associated with the big changes taking place in the brain. To better forecast the possibility of dementia, Waisman researcher and Associate Professor of medical physics and psychiatry Bradley Christian is using MRI and PET scanning techniques to identify changes in the brain that can predict which adults with Down syndrome are susceptible to dementia. Although dementia is a high risk for many with Down syndrome, Waisman Center researcher and Assistant Professor of human development and family studies Sigan Hartley is working with Christian to document the neuropsychological changes that are associated with the changes in the brain documented by the MRI and PET scans. Together these two researchers are helping to identify pathways to healthy aging in people with Down syndrome.
Making Medicine
Taking scientific discovery to the clinic is no small task. Among the many hurdles any new therapy must clear is devising a way to manufacture agents – drugs, vaccines, vectors, cells – for clinical trials in a way that meets strict FDA standards. In 2001, Waisman Biomanufacturing was set in motion under the direction of Derek Hei and over the past decade has developed a portfolio of clinical products, including some of the first therapeutic human embryonic stem cells as well as vaccines, recombinant proteins and products for gene therapy. With seven cleanroom areas capable of clinical-quality production of a suite of biological therapeutic agents, the Waisman Center has partnered widely with companies in Wisconsin, the United States and abroad, as well as with academic research centers to provide novel agents used in medical trials.


